Paragon Medical offers concept development, product design, contract engineering, prototyping, testing, and contract manufacturing for medical devices.
We work with OEMs, entrepreneurs, venture capital, and angel investor backed companies to bring new medical devices and equipment to market. Have a concept? We’ll flesh it out. Have a design? We’ll help you prototype, test, and refine it. Have it all? We’ll help you produce it. Wherever you are in your product’s evolution, we’ll get you where you need to go.
Working together, we establish common goals – then we achieve them. Our innovative, high-value concept-to-supply services span design, development engineering, sourcing, assembly and packaging services. We offer:
- Medical device product design
- Medical device product development
- Contract engineering for medical devices
- Medical device design and product testing and validation
- Medical device contract manufacturing
Whether you’re in the concept, development, or production phase, Paragon Medical helps you bridge the milestones to medical device commercialization.
Ready to get started?
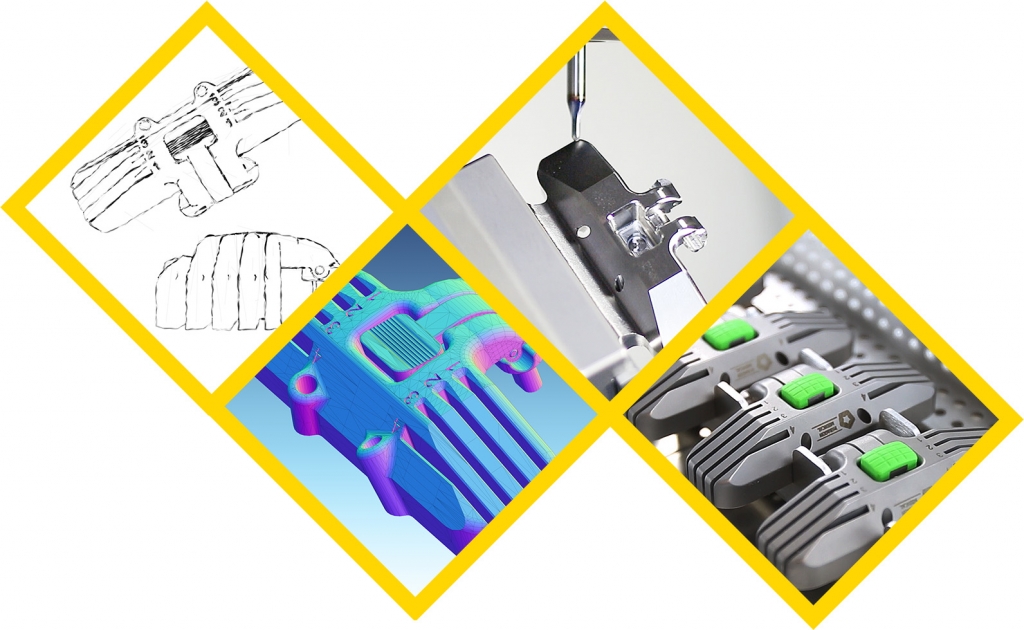
Why Choose Paragon’s Design & Development Services?
Most medical device and product development companies focus on a specific part of the process, specializing either in concept and design or in medical device contract manufacturing.
Paragon Medical is different. Formerly known as Bridgemedica, we are a long-term design and manufacturing partner that supports all phases of the medical device design, development, and manufacturing process, so you don’t have to split up your project at critical junctures.

With Paragon, you have a one-stop shop for all your design, testing, regulatory, and manufacturing needs that will help you:
Fill Competency Gaps
on your team with expert engineers, manufacturing processionals, and specialty capabilities.
Strengthen Your Intellectual Property
with technology evaluations and competitive analyses.
Minimize Risk
with ISO and QSR compliance, competitive costs, and regulatory process expertise.
Accelerate Time to Market
as our engineers refine design for manufacturability; develop scalable, repeatable manufacturing processes; and smoothly transition you from prototyping to production.
Reduce Manufacturing Costs
through design for manufacturability, lean manufacturing, and technology and process development.
Enable Vertical Integration
with a one-stop shop for design and development, tooling, component manufacturing, assembly, packaging, sterilization, distribution, and more.
Featured Resource: Why Partner with Paragon Medical?
From commercialization to world-class service, download the datasheet to learn more about how we can help you make a difference in the highly competitive healthcare market. Get your copy now.
Individual or Full Service Throughout the Medical Device Design & Development Phases
Our team has extensive project management experience through the entire commercialization process, and we’ve designed our modular Design & Development services to provide maximum flexibility.
Use our full service to move through all the phases below, or work with us for just one or two phases. Regardless of when you come to us, we’ll get you to the finish line.
Talk to an expert about how we can help accelerate your project through its current stage.
Phase 1: Medical Device Concept Development
In Phase 1, we begin with a concept or a problem. Our team of medical device designers and engineers conducts research to gain an understanding of the market requirements and design input. We brainstorm multiple medical device concepts, then narrow them down.
Once the concept is built out, we provide medical device design services, the results of which are tested and validated through prototyping. With access to rapid prototyping technology, we can quickly put those prototype devices into the hands of the end user for feedback on feasibility. We iterate working models and can support market research and focus groups as needed.
Want to learn more about our capabilities? Download our brochure on Rapid and Quick Turn Prototyping.

Phase 2: Development
During phase two, we develop a full project plan with formal design controls. We refine the conceptual medical device design through additional iterations and establish detailed engineering specifications and tolerances. Our engineers will initiate hazard analysis on the design and begin development of test methods.
By the end of phase 2, the design form has been selected and critical performance requirements tested.

Phase 3: Verification/Validation
In phase 3, we finalize test methods for the new medical product design. Design verification testing ensures performance meets design input objectives. Design validation testing is performed under defined operating conditions (either actual or simulated use) on initial production units or equivalents.
We also complete product packaging design is completed and biocompatibility testing in this phase.

Phase 4: Manufacturing Scale-Up
Our scale-up includes process validation, process capability and sterility validation. This testing is performed on product built under full manufacturing level documentation.
Suppliers are qualified and production equipment is installed and qualified. Shelf life requirements are met before phase 4 is complete. Final documentation is also established, including labeling and instructions for use.

Phase 5: Full-Scale Manufacturing
During phase 5, we secure all regulatory approvals and carefully train staff to implement the full production of your medical device product.
We understand the compliance process, including regulatory and design validation requirements. This knowledge allows for a more seamless design transfer to ensure a timely launch.
We build the inventory to support product launch and provide timely responses to your product forecasts. For more information on how we can ensure efficient, effective, and proactive responses to your product demand forecasts, read about our Sales & Operations Planning services.

Design & Development Services Benefits
Paragon operates as an extension of your development team and can support you from the very inception of your project. We partner with our customers by providing premier engineering from start to finish, developing value engineered products. We have all the necessary resources to integrate our activities into our client’s product development and supply chain process so our clients can realize substantial benefits.
Reduce Manufacturing Costs
A key benefit of our design and development services is the teamwork between design engineers and manufacturing experts. Our capabilities cover a broad spectrum of medical device products and our team members have extensive experience in design, development, and manufacturing of medical devices, giving us a first-hand understanding of the industry’s product development needs. We’re well versed in the latest technology, including usability, software, and equipment.
With deep experience in both the front-end design phases and the back-end manufacturing phases, our team can design high performing medical devices that can also be efficiently and cost-effectively manufactured. Our design and design for manufacturability expertise can reduce complexity that results in the reduction of both cost and quality issues while ensuring long-term, efficient manufacturing operations.
Working as an extension of your business’s R&D department, we rapidly develop a meticulous plan that minimize steps but optimizes material yield. From recommendations for tolerances that improve the ease and speed of manufacturing, we ensure proper function that results in faster, more cost-effective delivery of high-quality products.
Accelerate Time to Market and Minimize Risk
How many products do you have in development? How many instruments must be concepted, prototyped, and validated? Involve us in your product development and we can help you get more done in less time.
Our turnkey concept-to-supply business model accelerates time-to-market and minimizes risk for our clients. With ISO and QSR compliance, state-of-the-art facilities, competitive costs, and an established network of global contacts including the Far East, we can get you there.
We always have your finish line in mind. We respond promptly to your needs and can adjust planning and execution rapidly to accommodate any changes that may arise. Our understanding of the regulatory demands as well as the entire commercialization process affords our clients the confidence that their product will launch on time.
Strengthen Intellectual Property and Gain Technology Expertise
We know we’re not the only resource working to move your project forward. There are other important capabilities that can make the difference in your program. We provide technology evaluation and assessment, competitive analysis, and services for intellectual property generation and landscape.
We can help you strengthen your intellectual property portfolio or create a matrix of existing art to determine opportunities and points of weakness. Our industry experience ensures a thorough assessment and we can offer distribution of your company’s products across all medical device markets.
In addition, we provide design control, compliance related documentation tracking, regulatory filings and domestic and offshore sourcing of components and subassemblies.
An Experienced Medical Device Design Company
We’ve helped entrepreneurs, OEMs, and startup companies with a wide range of medical device designs and product manufacturing. Below are just a few of the medical product design types we’ve worked on.
- Drug delivery device designs
- Biomedical device designs
- Orthopaedic surgical instrument designs
- Handheld biopsy device designs
- Endoscopy device designs
- Implantable component designs
- LVAD product designs
As one of few medical device contract manufacturing companies that can help with product design, we can support your design phase all the way through to commercialization.
CONTACT



